The dissolution of the slightly soluble salt CaF2 is a fundamental process in chemistry with significant implications in various industries and environmental contexts. Understanding the factors governing the solubility of CaF2 is crucial for optimizing its applications and mitigating potential environmental concerns.
This article explores the concept of slightly soluble salts, the dissolution equilibrium of CaF2, the influence of temperature, pH, and common ion effects on its solubility, and the practical applications of CaF2 in industries such as glass and ceramic production.
Dissolution of Slightly Soluble Salt CaF2
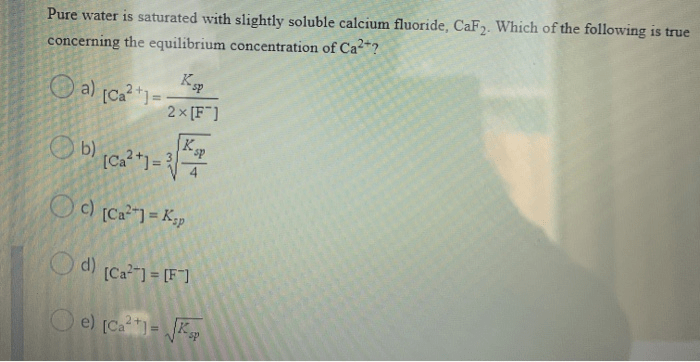
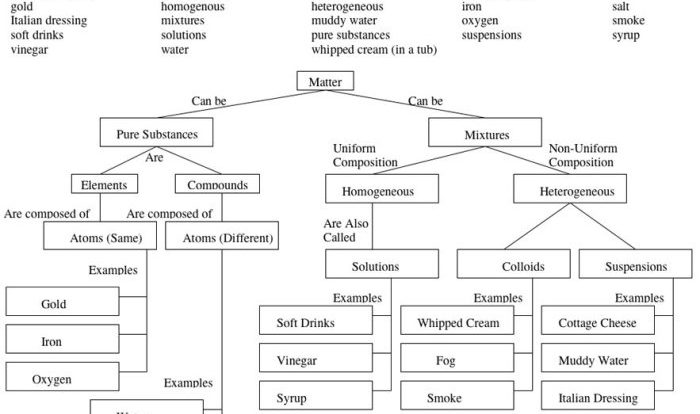
Slightly soluble salts are those that dissolve in water to a limited extent, resulting in a saturated solution. CaF2, calcium fluoride, is an example of a slightly soluble salt.
The dissolution of CaF2 in water can be represented by the following chemical equation:
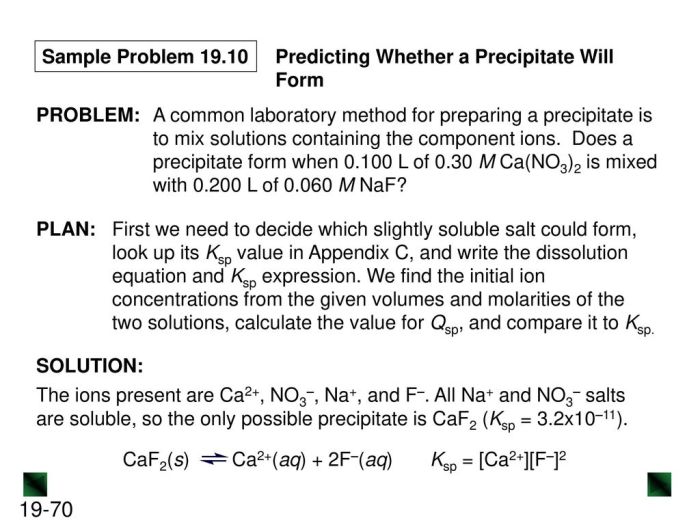
CaF2(s) + H2O(l) ⇌ Ca2+(aq) + 2F-(aq)
The solubility of CaF2 is influenced by several factors, including temperature and pH. Higher temperatures generally increase the solubility of slightly soluble salts, while changes in pH can affect the equilibrium of the dissolution process.
Equilibrium and Solubility Product, Dissolution of the slightly soluble salt caf2
Chemical equilibrium occurs when the forward and reverse reactions of a chemical process occur at the same rate, resulting in no net change in the concentrations of the reactants and products.
The solubility product (Ksp) is a constant that represents the equilibrium constant for the dissolution of a slightly soluble salt. For CaF2, the Ksp is given by:
Ksp = [Ca2+][F-]^2
The Ksp value for CaF2 is 3.16 x 10^-11, indicating that the dissolution of CaF2 is a slightly exothermic process.
Common Ion Effect
The common ion effect is a phenomenon that occurs when a common ion is added to a solution containing a slightly soluble salt. The addition of a common ion shifts the equilibrium of the dissolution process towards the solid phase, decreasing the solubility of the salt.
For CaF2, the addition of Ca2+ or F- ions to the solution will decrease its solubility. This is because the added ions compete with the Ca2+ and F- ions from the dissolution of CaF2 for solvation, reducing the concentration of the dissolved ions and shifting the equilibrium towards the solid phase.
Applications and Industrial Relevance
CaF2 has various applications in industries such as glass and ceramic production, where it is used as a flux to lower the melting point of the materials.
The dissolution of CaF2 is also utilized in the production of hydrofluoric acid (HF), an important industrial chemical used in the etching of glass and the production of fluorocarbons.
However, the dissolution of CaF2 can also have environmental implications, as the release of fluoride ions into water sources can potentially lead to fluorosis in humans and animals.
FAQ Compilation: Dissolution Of The Slightly Soluble Salt Caf2
What is the solubility product (Ksp) of CaF2?
The Ksp of CaF2 is 3.16 x 10^-11, indicating its low solubility in water.
How does temperature affect the solubility of CaF2?
The solubility of CaF2 increases with increasing temperature, as higher temperatures favor the dissolution process.
What is the common ion effect?
The common ion effect states that the presence of a common ion in solution decreases the solubility of a sparingly soluble salt by shifting the equilibrium towards the solid phase.