Synthesis of an alum lab – Embark on a fascinating journey into the realm of alum synthesis! This comprehensive guide will unravel the historical significance, industrial applications, and step-by-step procedures for crafting this remarkable compound in your lab. Prepare to be captivated as we delve into the intricacies of alum synthesis, leaving no stone unturned.
From its humble beginnings to its widespread use in industries like water treatment and medicine, alum holds a rich history and practical significance. Join us as we explore the various methods of alum synthesis, examining their advantages and disadvantages to equip you with the knowledge to choose the most suitable approach for your research or educational endeavors.
Introduction to Alum Synthesis
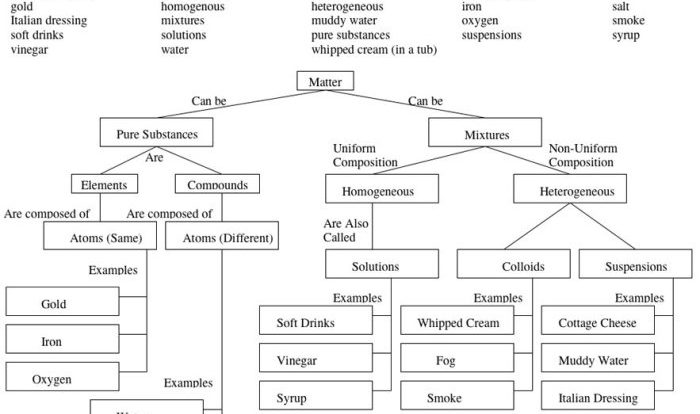
Alum is a general term for a class of inorganic chemical compounds known as hydrated metal sulfates with the general formula M(I)M(III)(SO 4) 2·12H 2O, where M(I) is a monovalent cation such as potassium or ammonium, and M(III) is a trivalent cation such as aluminum or iron.Alum
has a long and storied history, dating back to ancient times. It was first used as a mordant, a substance used to fix dyes to fabrics. Alum was also used in the production of paper, leather, and glass. In the Middle Ages, alum was used in the production of gunpowder.Today,
alum is still used in a variety of industries, including the food, pharmaceutical, and water treatment industries. It is also used as a coagulant in the production of paper and textiles.
Historical Significance of Alum
Alum has been used for centuries in a variety of applications, including:
- As a mordant in the dyeing of fabrics
- In the production of paper
- In the production of leather
- In the production of glass
- In the production of gunpowder
Alum was first used in ancient China, where it was used as a mordant in the dyeing of fabrics. Alum was also used in the production of paper in China, and this technology was later adopted by the Arabs and Europeans.In
the Middle Ages, alum was used in the production of gunpowder. Gunpowder was first invented in China, and it was later adopted by the Arabs and Europeans. Alum was used as an oxidizing agent in gunpowder, and it helped to create a more powerful explosive.Alum
continued to be used in a variety of applications throughout the Renaissance and the Industrial Revolution. It was not until the 19th century that the chemical composition of alum was finally determined.
Importance of Alum in Various Industries
Alum is still used in a variety of industries today, including:
- The food industry
- The pharmaceutical industry
- The water treatment industry
- The paper industry
- The textile industry
In the food industry, alum is used as a coagulant in the production of cheese and other dairy products. It is also used as a stabilizer in the production of canned fruits and vegetables.In the pharmaceutical industry, alum is used as an astringent and an antiseptic.
It is also used in the production of vaccines and other medical products.In the water treatment industry, alum is used as a coagulant in the removal of impurities from water. It is also used to remove fluoride from water.In the paper industry, alum is used as a sizing agent.
Sizing agents help to make paper more resistant to water and ink.In the textile industry, alum is used as a mordant in the dyeing of fabrics. It is also used to make fabrics more flame-resistant.
Methods of Alum Synthesis
Alum synthesis encompasses various methods, each with its advantages and disadvantages. These methods can be broadly classified into two main categories: wet methods and dry methods.
Wet Methods
Wet methods involve the reaction of aluminum salts with sulfuric acid or ammonium sulfate in aqueous solutions. These methods typically produce high-purity alum crystals.
- Double Decomposition Method:This method involves the reaction of an aluminum salt (e.g., aluminum sulfate) with ammonium sulfate in aqueous solution. The reaction results in the formation of alum crystals and a precipitate of aluminum hydroxide.
- Sulfuric Acid Method:This method involves the reaction of an aluminum salt (e.g., aluminum hydroxide) with sulfuric acid in aqueous solution. The reaction produces alum crystals and releases water.
Dry Methods
Dry methods involve heating a mixture of aluminum oxide and ammonium sulfate in the absence of water. These methods are less commonly used due to the potential for impurities and the difficulty in controlling the reaction conditions.
- Thermal Decomposition Method:This method involves heating a mixture of aluminum oxide and ammonium sulfate at high temperatures. The reaction produces alum crystals and releases ammonia and water.
| Method | Reactants | Products |
|---|---|---|
| Double Decomposition Method | Aluminum salt, Ammonium sulfate | Alum crystals, Aluminum hydroxide |
| Sulfuric Acid Method | Aluminum salt, Sulfuric acid | Alum crystals, Water |
| Thermal Decomposition Method | Aluminum oxide, Ammonium sulfate | Alum crystals, Ammonia, Water |
Materials and Equipment Required

To embark on the synthesis of alum, a meticulous assembly of essential materials and equipment is indispensable. This section will elucidate the necessary components and their indispensable roles in the experimental process.
The materials and equipment can be conveniently categorized into two groups: chemicals and apparatus. Each item plays a distinct role in facilitating the successful synthesis of alum, as detailed in the table below.
Chemicals
- Aluminum sulfate: A crucial reactant, providing the aluminum ions required for alum formation.
- Potassium sulfate: Another essential reactant, supplying the potassium ions necessary for alum formation.
- Sodium hydroxide: A strong base used to adjust the pH of the reaction mixture.
- Water: An essential solvent for the reaction and crystallization process.
Apparatus
- Beakers: Used to hold the reaction mixture and solutions.
- Stirring rod: Facilitates the mixing of the reaction mixture.
- Filter paper: Used to filter the precipitated alum crystals from the reaction mixture.
- Thermometer: Monitors the temperature of the reaction mixture.
- Hot plate: Provides heat for the reaction mixture.
- Evaporating dish: Used to evaporate the excess water from the alum solution.
li> Funnel: Assists in the filtration process.
Step-by-Step Synthesis Procedure

Let’s delve into the practical steps involved in synthesizing alum. Safety should be our top priority throughout the process.
Remember to wear appropriate safety gear, including gloves, goggles, and a lab coat. Conduct the synthesis in a well-ventilated area to avoid inhaling harmful fumes.
Materials
- Potassium aluminum sulfate dodecahydrate (AlK(SO4)2·12H2O)
- Aluminum sulfate octadecahydrate (Al2(SO4)3·18H2O)
- Water
Equipment
- Beaker
- Stirring rod
- Hot plate
- Thermometer
- Filter paper
- Funnel
- Evaporating dish
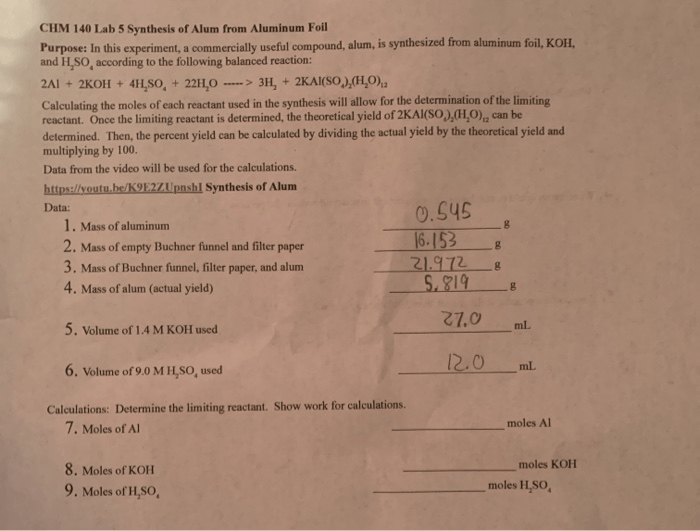
Procedure, Synthesis of an alum lab
- In a beaker, dissolve 50 g of potassium aluminum sulfate dodecahydrate and 100 g of aluminum sulfate octadecahydrate in 200 mL of water.
- Heat the solution on a hot plate with constant stirring until it reaches 90°C.
- Maintain the temperature at 90°C for 30 minutes, stirring occasionally.
- Allow the solution to cool slowly to room temperature.
- Filter the solution through filter paper into an evaporating dish.
- Allow the filtrate to evaporate slowly in a well-ventilated area.
- Once the crystals have formed, collect them and dry them on filter paper.
Chemical Reactions Involved
Alum synthesis involves a series of chemical reactions that lead to the formation of the desired product. These reactions can be divided into two main stages: the formation of aluminum hydroxide and the subsequent reaction with potassium sulfate to form alum.
Formation of Aluminum Hydroxide
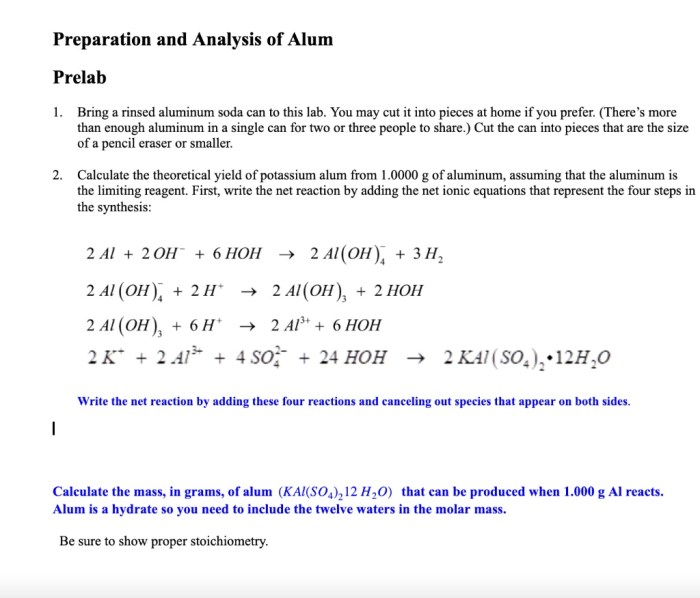
The first stage of the synthesis process involves the reaction of aluminum metal with sodium hydroxide. This reaction produces aluminum hydroxide and hydrogen gas, as shown in the following balanced chemical equation:
Al(s) + 6NaOH(aq) → 2Na3AlO 3(aq) + 3H 2(g)
In this reaction, aluminum atoms are oxidized from a neutral state to a +3 oxidation state, while sodium atoms are reduced from a +1 oxidation state to a neutral state. The aluminum hydroxide formed in this reaction is a white, gelatinous precipitate.
The synthesis of an alum lab involves a series of chemical reactions that result in the formation of potassium aluminum sulfate dodecahydrate. If you’re looking for more information on the subject, check out the final exam for is 100 c . The lab itself is relatively straightforward, but it does require careful attention to detail.
By following the instructions carefully, you can ensure that your alum crystals turn out perfectly.
Reaction with Potassium Sulfate
The second stage of the synthesis process involves the reaction of aluminum hydroxide with potassium sulfate. This reaction produces alum and potassium hydroxide, as shown in the following balanced chemical equation:
Na3AlO 3(aq) + K 2SO 4(aq) → KAl(SO 4) 2·12H 2O(s) + NaOH(aq)
In this reaction, aluminum atoms remain in the +3 oxidation state, while potassium atoms remain in the +1 oxidation state. The alum formed in this reaction is a white, crystalline solid.
Purification and Crystallization of Alum: Synthesis Of An Alum Lab

Purification and crystallization are crucial steps in obtaining pure and well-defined alum crystals. These processes remove impurities and promote the formation of large, well-shaped crystals.
Several methods are employed for purifying and crystallizing alum:
Recrystallization
Recrystallization involves dissolving the impure alum in a suitable solvent, filtering the solution to remove impurities, and then allowing the purified solution to slowly crystallize.
The solvent used for recrystallization should be one in which the alum is soluble at high temperatures but less soluble at low temperatures. Common solvents include water, ethanol, and acetone.
The process of recrystallization can be repeated multiple times to further purify the alum crystals.
Sublimation
Sublimation is a process in which a solid directly transforms into a gas without passing through the liquid phase. In the case of alum, sublimation can be used to purify the crystals by heating them under vacuum.
The purified alum vapor condenses on a cooler surface, forming pure crystals.
Zone Refining
Zone refining is a technique used to purify materials by repeatedly melting and solidifying a narrow zone along the length of the material.
As the zone moves along the material, impurities are concentrated in the molten zone and are removed when the zone solidifies. This process can be repeated multiple times to achieve a high level of purity.
Crystallization from Solution
Crystallization from solution involves dissolving the alum in a suitable solvent and then slowly evaporating the solvent to promote crystal growth.
The rate of evaporation can be controlled by adjusting the temperature and humidity of the environment. Slow evaporation allows for the formation of larger and more well-defined crystals.
Applications of Alum

Alum, a versatile compound, finds applications in various industries due to its unique properties such as astringency, coagulating ability, and acidity. Its usefulness stems from its ability to form insoluble precipitates with impurities, clarify liquids, and neutralize alkalinity.
The following are some notable applications of alum:
Water Treatment
- Coagulant and Flocculant:Alum is widely used as a coagulant and flocculant in water treatment plants. It helps remove impurities, suspended solids, and microorganisms by forming insoluble precipitates that can be easily settled and filtered out.
- Removal of Phosphorus:Alum is effective in removing phosphorus from wastewater. Phosphorus is a nutrient that can contribute to eutrophication, leading to algal blooms and water quality degradation. Alum binds to phosphorus, forming insoluble precipitates that can be removed from the water.
Papermaking
- Sizing Agent:Alum is used as a sizing agent in papermaking. It helps improve the paper’s strength, water resistance, and resistance to ink penetration. Alum reacts with rosin, a natural resin, to form an insoluble precipitate that fills the pores of the paper, making it less absorbent.
Medicine
- Astringent:Alum is an astringent, which means it can cause tissues to contract. It is used as a topical treatment for minor cuts, burns, and skin irritations. Alum helps reduce inflammation and bleeding, and it can also help dry out weeping wounds.
- Antiperspirant:Alum is used as an active ingredient in some antiperspirants. It helps reduce sweating by blocking the sweat glands.
Detailed FAQs
What is the chemical composition of alum?
Alum is a double sulfate salt, typically composed of potassium aluminum sulfate dodecahydrate (KAl(SO4)2·12H2O) or ammonium aluminum sulfate dodecahydrate (NH4Al(SO4)2·12H2O).
What are the historical uses of alum?
Alum has been used for centuries in various applications, including as a mordant in dyeing, a coagulant in water purification, and an astringent in medicine.
What are the industrial applications of alum?
Alum is widely used in water treatment plants, papermaking, leather tanning, and textile manufacturing.